As of January 26, 2026
Documented Breakthrough COVID-19 Cases: 496
Latest positive test for a breakthrough infection: January 19, 2025;
Last reported side effects from booster: October 6 2025(LP.8.1 strain)
Latest breakthrough COVID-19/Long COVID fatality:
August 13, 2023 (70-79 age bracket); February 28, 2023 (50-59 age bracket)
Latest supercentenarian COVID-19 fatality:
January 3 2023 (110+ age bracket)
Oldest COVID-19-survivor recently passed: Maria Branyas Morera, born 4 March 1907, COVID in April 2020,
2 doses of vaccine in January 2021; died on 19 August 2024 at the age of 117 years, 168 days
Maria Mochi: COVID at 106 and 109; died at the age of 110 years, 250 days.
Oldest COVID-19-survivor:
Lucile Randon (Sister André)
survived COVID-19 just before her 117th birthday. She died two years later, on 17 January 2023, at the age of 118 years, 340 days
A young woman that had systematic COVID 11 times
Peer-Reviewed Publication: Gabashvili IS. The Incidence and Effect of Adverse Events Due to COVID-19 Vaccines on Breakthrough Infections: Decentralized Observational Study With Underrepresented Groups. JMIR Form Res. 2022 Nov 4;6(11):e41914. doi: 10.2196/41914. PMID: 36309347; PMCID:
PMC9640199
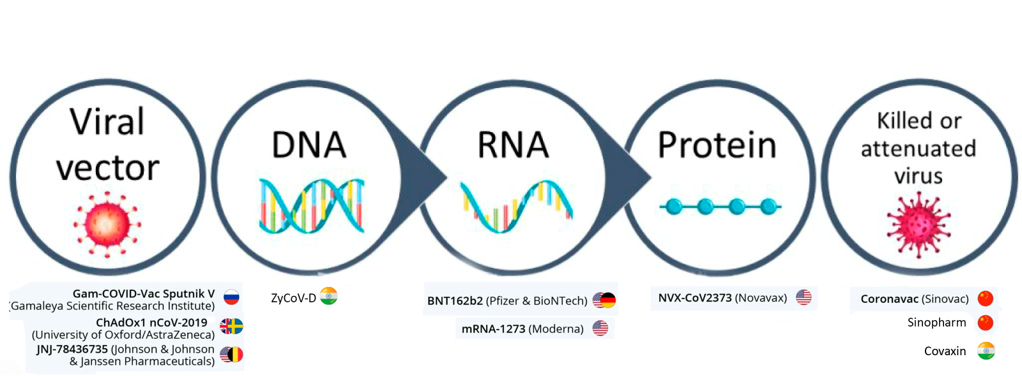
Vaccines
RNA
Non Replicating Viral Vector
Killed/Inactivated virus
Protein subunit